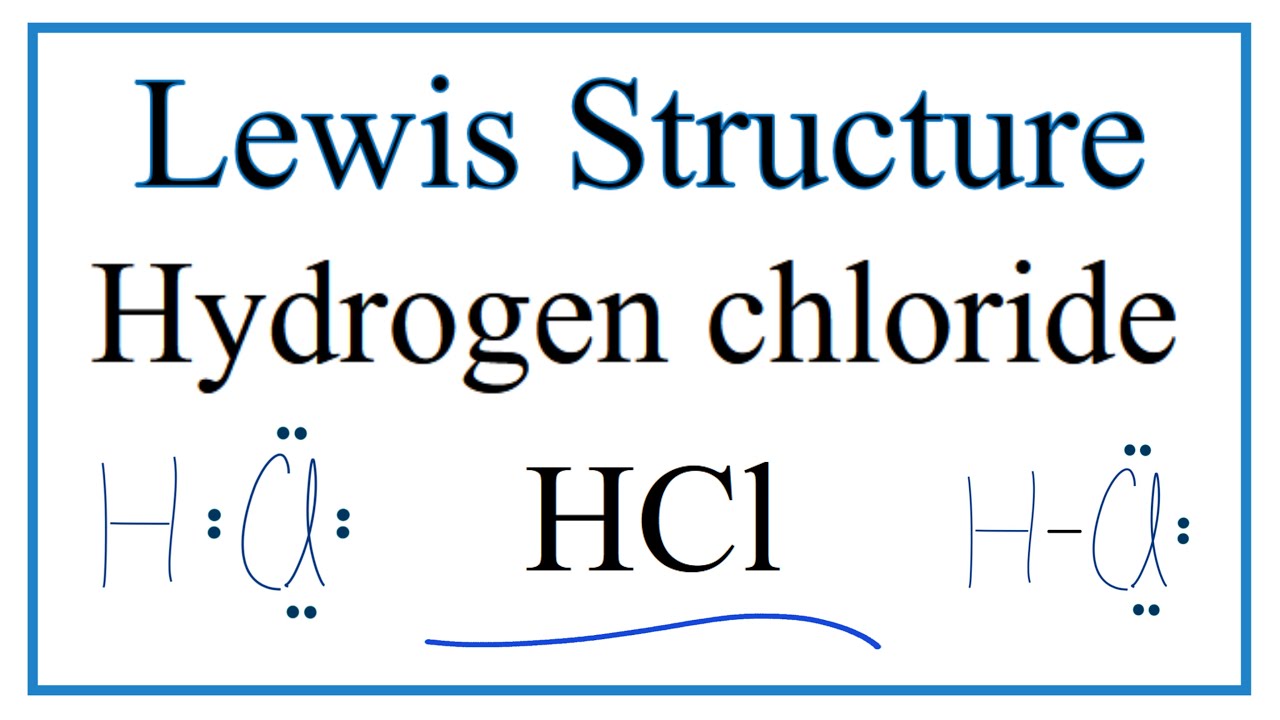
Hydrogen chloride bonding hcl molecule q6 Hcl hydrogen chloride covalent bonding dot cross diagram science Methane igcse dot cross chemistry covalent diagrams carbon dioxide ethane nitrogen hydrogen molecules use ethene bonds oxygen ammonia represent water dot and cross diagram for hydrogen chloride
IGCSE Chemistry: Covalent Substances (Section 1g)
As chemistry foundation bonding and structure page Hydrogen chloride: dot and cross diagram for hydrogen chloride Hydrogen chloride diagram chlorine electron dot cross arrangement covalent bonding diagrams hcl electrons compounds shell show outer bond correct chemistry
Dot chloride hydrogen bonding hcl molecules q3
Covalent dot-cross diagramsHow to draw the lewis dot structure for hydrogen chloride Igcse chemistry: covalent substances (section 1g)Igcse chemistry 2017: 1.46: understand how to use dot-and-cross.
Hydrogen covalent dot cross bonding diagrams chlorine electron oxygen atoms chloride h2 molecule between two sharing cl2 compounds nitrogen carbonDot and cross diagrams of simple molecules 1.39 explain, using dot and cross diagrams, the formation of covalentBonding covalent structure chlorine molecule atoms chloride electrons two hydrogen bond cl2 chemistry sharing pair non formed gif c3 venn.
Oxygen dot cross bonding diagrams covalent electrons chlorine bbc electron compounds sharing formation hydrogen atom water gas atoms elements chemistry
1.39 explain, using dot and cross diagrams, the formation of covalentDot cross diagram cl2 chlorine covalent chloride diagrams hydrogen iodine cl gas Hydrogen chloride: dot and cross diagram for hydrogen chlorideDot and cross diagrams of simple molecules.
Hydrogen cross chloride cyanide sodiumHydrogen chloride n2 nitrogen chlorine reacts sodium Hcl hydrogen chloride covalent bonding bw secondaryDot cross chlorine chemistry hydrogen diatomic oxygen igcse nitrogen diagrams halogens covalent represent bonds ammonia halogen.

Igcse chemistry 2017: 1.46: understand how to use dot-and-cross
Lewis structure chloride dot hydrogen draw .
.